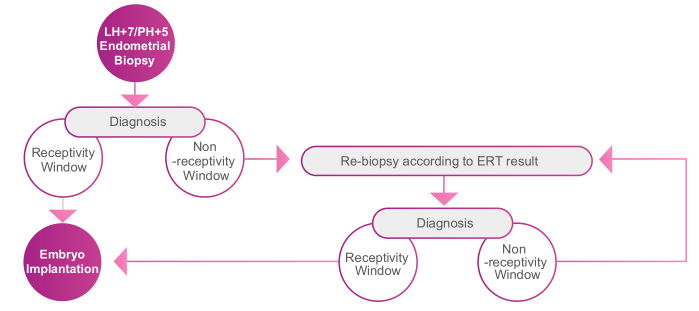
ERT (Endometrial Receptivity Test) is to determine endometrial receptivity by RNA-seq analysis of the expression levels of numerous genes.

Embryo implantation is a complex process involving many biological mechanisms, in which endometrial receptivity and embryo quality are the most influential factors. 1/3 implantation failure is due to the low quality of the embryo and the other 2/3 is due to insufficient endometrial receptivity. Thus, finding the right window of endometrial receptivity for embryo implantation can greatly improve the clinical pregnancy of IVF-ET (In vitro Fertilization & Embryo Transfer).
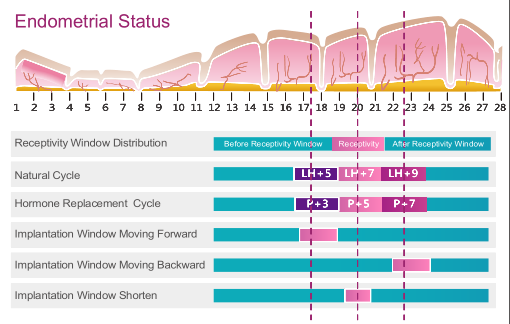
ERT can be used for both natural reproduction cycles and hormone replacement therapy cycles. Endometrial tissue is collected through biopsy at LH+7 or P+5. The date of Embryo transfer is determined/adjusted according to ERT results.
Who is it for?
This study is aimed at patients with repeated implantation failures (3 or more failures with embryos of quality, or failures with implantation of at least 10 available embryos). The conditions including uterus pathological diseases, thrombosis inclination, repeated pregnancy loss, chromosomal abnormalities of the embryo should be excluded.