Kit para la detección de la mutación G20210A del gen de la protrombina mediante PCR en tiempo real, utilizando la tecnología de sondas TaqMan®
Información del producto
La protrombina (factor II de coagulación) es una glicoproteína sintetizada en el hígado. Es un componente esencial del mecanismo de coagulación. La regulación de la expresión de la protrombina es crucial para el mantenimiento de la homeostasis.
La mutación G20210A del Factor II presenta una alta prevalencia (18%) en familias con trombosis y en pacientes como primer caso familiar de la enfermedad (6,2%). Esta mutación causa un aumento en los niveles de protrombina, lo que conduce a un elevado riesgo de trombosis.
Uso previsto
Genvinset® Factor II G20210A es un kit de diagnóstico in vitro semiautomatizado para la determinación cualitativa de la mutación G20210A (NCBI dbSNP rs1799963; NM_000506.5:c.*97G>A) en el gen de la protrombina (FII) (OMIM: 176930) asociada con el riesgo de trombosis, en muestras de ADN genómico extraído de sangre total, mediante tecnología real-time PCR usando sondas TaqMan®.
Los pacientes que pueden beneficiarse de esta determinación son los remitidos por un especialista. Los resultados de este ensayo no deben ser los únicos en los que debe basarse la decisión terapéutica y deben ser utilizados como ayuda en el diagnóstico junto con resultados de otros marcadores de la enfermedad.
El usuario previsto del kit es personal técnico entrenado y cualificado para realizar el protocolo y la interpretación de los resultados descritos en las instrucciones de uso.
Flujo de trabajo

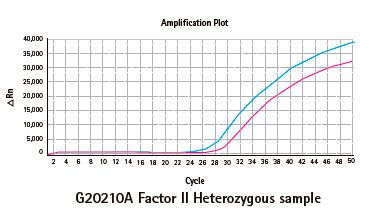
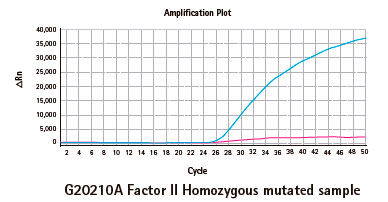
Resultados

Limitaciones
- Las mutaciones o polimorfismos en los sitios de hibridación de los primers/sondas son posibles y pueden dar lugar a la falta de definición de alelos. Otras tecnologías podrían ser necesarias para resolver el tipaje.
- Los datos y la interpretación de los resultados deben ser revisados por personal cualificado.
- Este producto es una herramienta auxiliar para el diagnóstico de pacientes con sospecha de trombofilia. Utilice estos resultados junto con los datos clínicos y los resultados de otras pruebas realizadas en el paciente.