Kit for detecting HLA-B*27 alleles by Real time PCR using TaqMan® probes technology
Information about the product
The major histocompatibility complex (MHC) is the genetic region that contains the most polymorphic loci of the human genome. It is involved in the mechanism of antigen presentation and, as such, defines the general immunological response.
Within the MHC, the allelic family HLA-B*27 is part of the HLA-B locus. The frequency of individuals carrying HLA-B*27 varies widely across populations, being approximately 7-9% in Caucasians.
The first leukocyte antigen (HLA) haplotype association with human inflammatory disease was discovered in 1972, correlating HLA-B*27 with ankylosing spondylitis (AS). This remains one of the strongest known relationships between a major histocompatibility complex antigen and a disease. HLA-B*27 alleles are present in up to 90% of patients in the majority of ethnic groups that suffer from AS.
In a similar way, high prevalence of this antigen was shown in other HLA-B*27 associated disorders including reactive arthritis (previously referred to as Reiter syndrome) (60-70%), psoriatic arthritis (14-40%), juvenile enthesitis related arthritis (60-90%) and acute anterior uveitis (50%).
Intended use
Genvinset® HLA B27v5 is a semi-automated in vitro diagnostic kit for HLA-B*27 group of alleles qualitative detection in genomic DNA extracted from whole blood, associated with ankylosing spondylitis predisposition, by Real-Time PCR using TaqMan® probes technology.
Patients who can benefit from this determination are those referred by a specialist with clinical symptoms compatible with rheumatoid diseases such as ankylosing spondylitis, reactive arthritis, psoriatic arthritis or juvenile enthesitis related arthritis, and acute anterior uveitis. The results of this test should not be the only ones on which the therapeutic decision is based and should be used as an aid in the diagnosis together with results of other markers of the disease.
The intended user of the kit is technical personnel trained to carry out the protocol and the interpretation of results described in the Instructions for Use.
Workflow

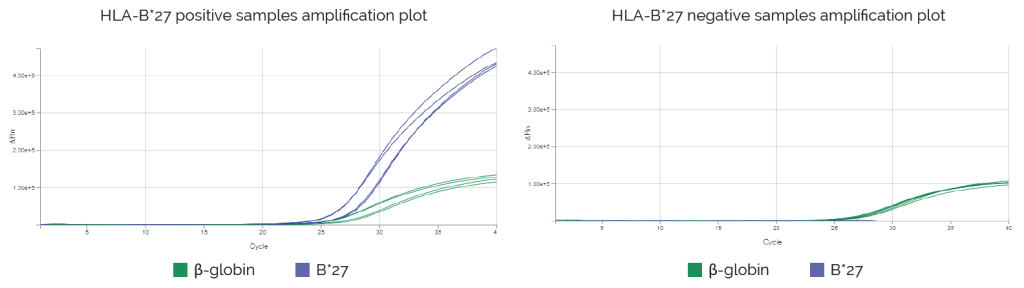
Results
Limitations
- Mutations or polymorphisms at annealing primer/probe sites are possible and may result in the lack of allele definition. Other technologies could be necessary to resolve the typing.
- Data and result interpretation should be revised by qualified personnel.
- This product is an auxiliary tool for the diagnosis of patients with suspected ankylosing spondylitis. Use these results in conjunction with clinical data and results of other tests performed on the patient.