myChoice CDx es la primera prueba de diagnóstico in vitro, aprobada por la FDA, que permite determinar el estado de deficiencia de la recombinación homóloga (HRD, por sus siglas en inglés), mediante NGS, en pacientes con cáncer de ovario.
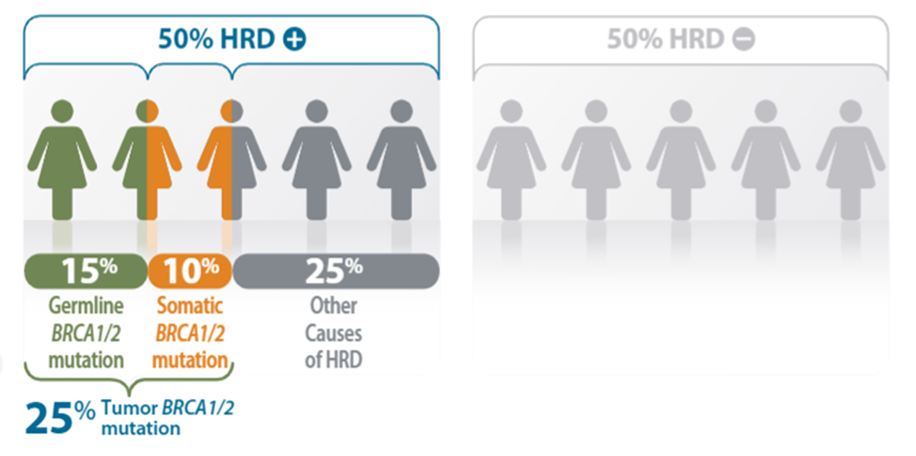
La recombinación homóloga es el mecanismo mediante el cual se reparan los daños en el ADN. Cuando la recombinación homóloga no es capaz de arreglar el ADN dañado, se habla de deficiencia de la recombinación homóloga. Aproximadamente el 50% de todos los casos de cáncer de ovario presentan HRD. El estado HRD se determina por el estado mutacional de los genes BRCA1 y BRCA2 y una medición de la inestabilidad genómica (GIS, genomic inestability status). Si hay mutación en los genes BRCA1 o BRCA2, o la medida de inestabilidad genómica GIS está por encima de un determinado nivel (42), se considera HRD positivo (HRD+).
Si el tumor es HRD+, es más probable que responda a agentes que bloquean la reparación del ADN, como los inhibidores de PARP (Poli-ADP ribosa polimerasa) (Olapariby Bevacizumab) que aquellos tumores sin inestabilidad genómica. Cuando los daños en el ADN no pueden corregirse, las células cancerosas son eliminadas, y el crecimiento del tumor se ralentiza o incluso desaparece. De esta manera, con myChoice CDx es posible evaluar el beneficio de la terapia con inhibidores de PARP en las pacientes.
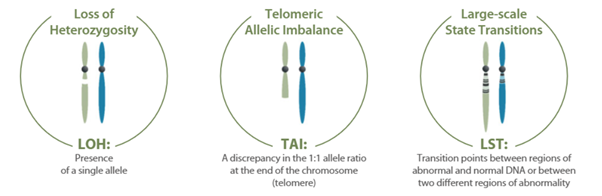
myChoice CDx detecta mediante NGS variantes de un único nucleótido (SNVs), inserciones/deleciones y grandes reordenamientos en las regiones codificantes y los límites intrón/exón de los genes BRCA1 y BRCA2. Además, realiza el cálculo de inestabilidad genómica (GIS) mediante un algoritmo que tiene en cuenta la pérdida de la heterocigosidad (LOH), el desequilibrio telomérico alélico (TAI) y las transiciones a gran escala (LST).
Se trata de la única prueba disponible comercialmente, diseñada para detectar grandes reordenamientos, que representan el 5% de todas las mutaciones del cáncer de ovario.
Características clave:
- Robustez
- Precisión
- Reproducibilidad
- Resultados de fácil interpretación