EndoPredict is a prognostic and predictive in vitro molecular test for patients with ER+/HER2- (estrogen receptor positive, human epidermal growth factor receptor 2 negative) primary breast cancer.
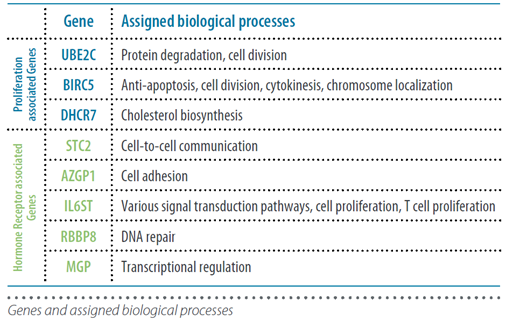
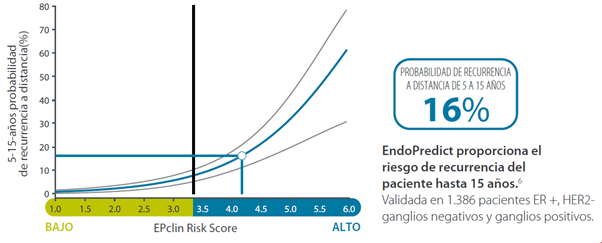
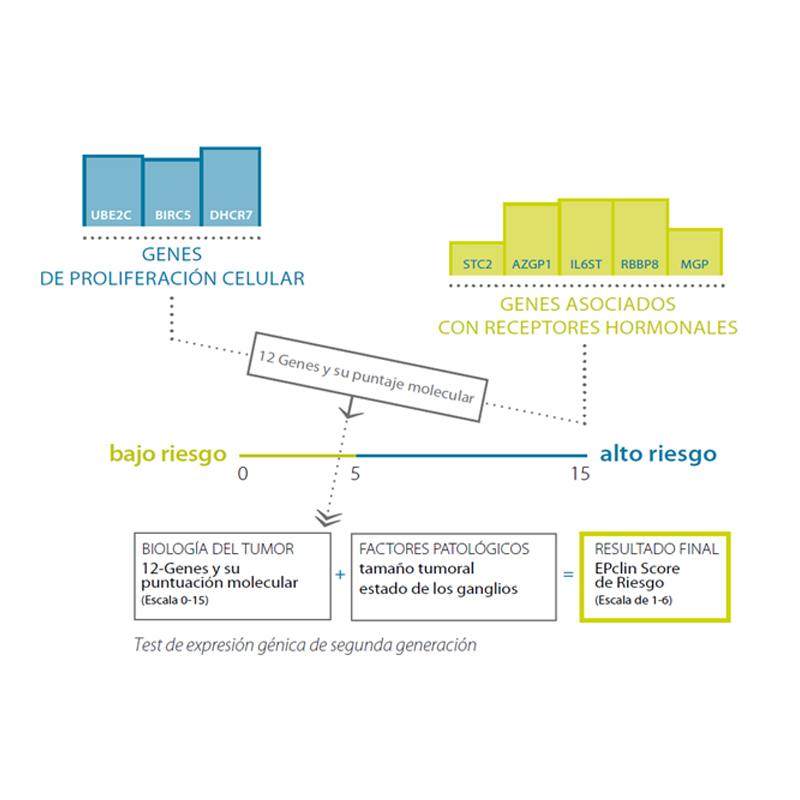
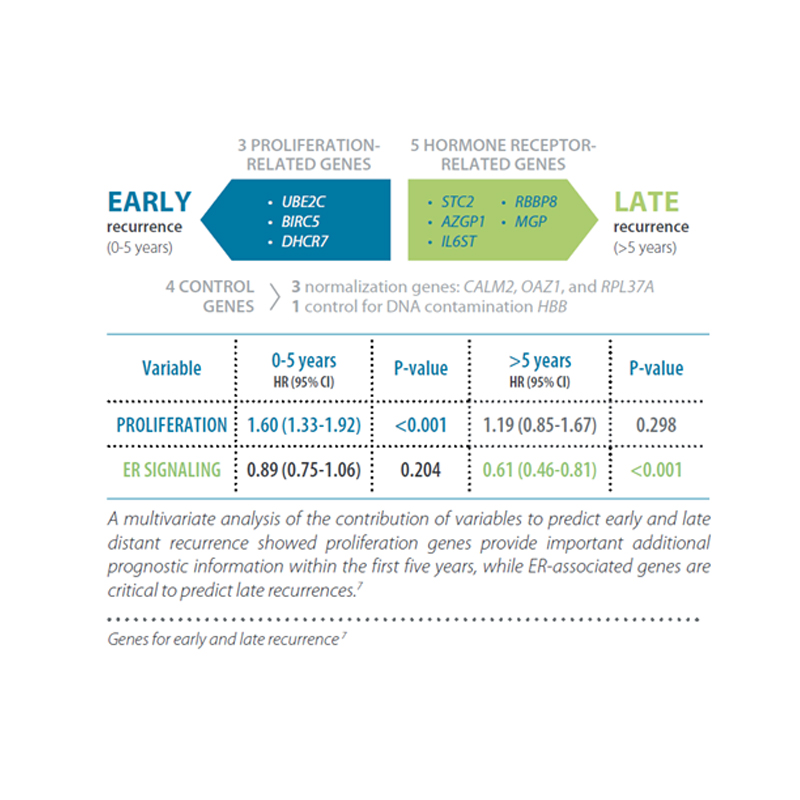
This second generation test combines a molecular score, obtained from the analysis of the expression of 12 genes (8 genes relevant to the course of the disease, 3 normalization genes and a control gene), with tumour size and nodal status (negative: N0, or positive: N+), resulting in the “EPclin Risk Score”. This score allows the establishment of a low-risk group or a high-risk group for recurrence.
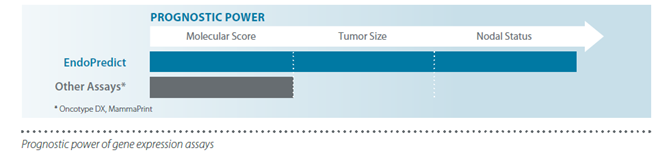
By combining the molecular score with clinical parameters (tumour size and nodal status), a prognostic accuracy superior to that of first generation tests is achieved, and it is possible to determine the risk of recurrence and the absolute benefit of chemotherapy over the next 15 and 10 years, respectively.
In this way, EndoPredict answers 3 important clinical questions:
- Can chemotherapy be avoided? – 10-year risk
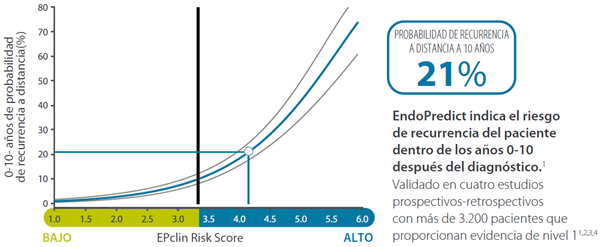
- What is the absolute benefit from chemotherapy at 10 years?
- Can endocrine therapy be stopped after 5 years? – Risk after 5 years and up to 15 years
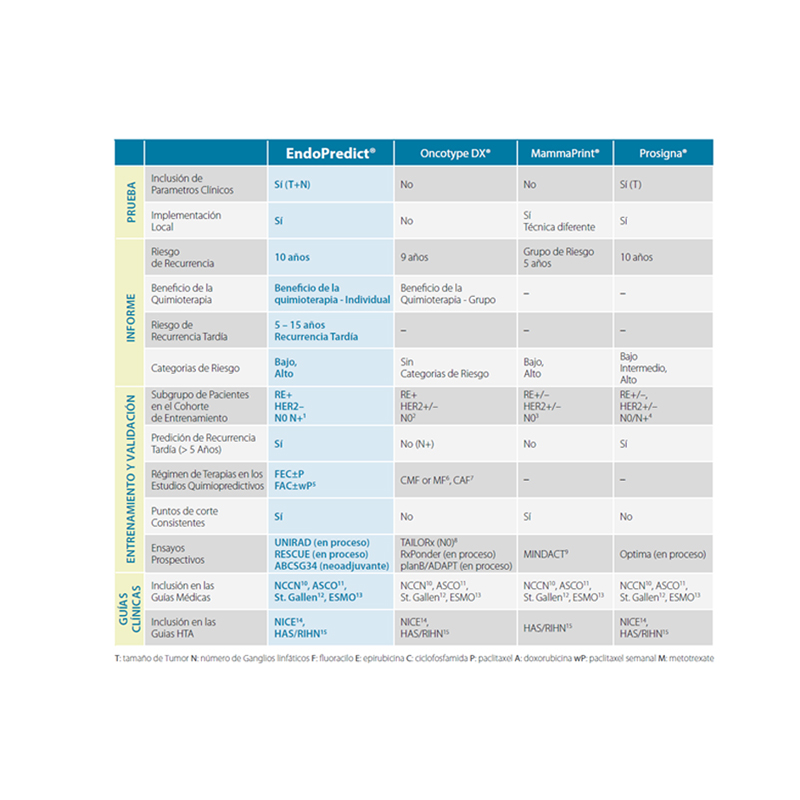
EndoPredict is recommended by international guidelines such as ASCO, ESMO, St. Gallen, EGMT, AJCC, AGO or NCCN, is being validated in four large clinical studies (with more than 3,200 patients) and used to guide treatment selection in more than 30,000 patients worldwide.
Key features:
• It is the only test that provides answers to these three questions: Can chemotherapy be avoided? What is the absolute benefit of chemotherapy? Can endocrine therapy be stopped after 5 years?
• It is the test that provides the largest group of “true” low-risk patients
• Second generation test, achieving superior prognostic accuracy
• Unique gene selection for accurate short- and long-term risk assessment
• Consistent study cohorts and constant cut-off point
• Clear low and high risk categories
• Fast results: within 2 days by local testing